IN VITRO RELEASE RATE FOR 1-MONTH
|
Drug (IVR formulation) |
In vitro release rate (mg day-1) |
|
TDF (TDF-FTC)
|
3.41 ± 0.33
|
|
FTC (TDF-FTC)
|
3.39 ± 0.84
|
|
TDF (TDF-FTC-MVC) |
1.59 ± 0.29
|
|
FTC (TDF-FTC-MVC) |
2.84 ± 0.17
|
|
MVC (TDF-FTC-MVC)
|
1.31 ± 0.17
|
IN VIVO RELEASE DATA IN MACAQUES
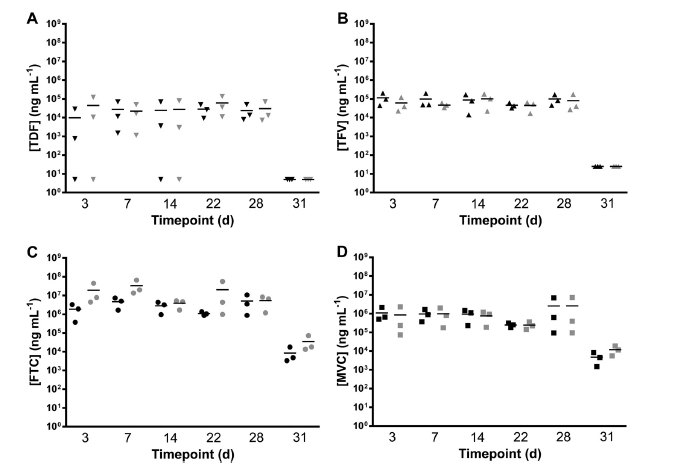
FIRST-IN-HUMAN TRIAL FOR HIV PROPHYLAXIS: VERSARING™ TRUVADA (TDF-FTC)
We are currently performing a clinical trial to test the safety and PK of three antiretroviral drug combinations (TDF, TDF-FTC and TDF-FTC-MVC) for the prophylaxis of HIV.
The TDF and TDF-FTC studies have been completed and show no significant safety concerns.
The IVRs were acceptable to all the six women in the trial.
The bioanalysis is in-progress.
