Endometriosis
MARKET POTENTIAL FOR AN INTRAVAGINAL RING FOR THE TREATMENT OF ENDOMETRIOSIS
-Approximately 10% of all American women in the reproductive age suffer from moderate to severe endometriosis. 20% of these women (1.2 million) are ideal candidates for treatment with gonadotropin-releasing hormones (GnRH) agonists such as leuprolide. Currently, these drugs are administered as monthly subcutaneous injections.
-Based on several analyst reports, we believe that the GnRH agonist market is greater than $1 billion in annual sales.
-Auritec Pharmaceuticals has formulated a GnRH agonist intravaginal ring (IVR) which may improve compliance and ease of use in women suffering from endometriosis and uterine fibroids. This IVR has been tested in vitro and in vivo and has shown sustained-release for 4 weeks. It is being further developed for a first-in-human clinical trial.
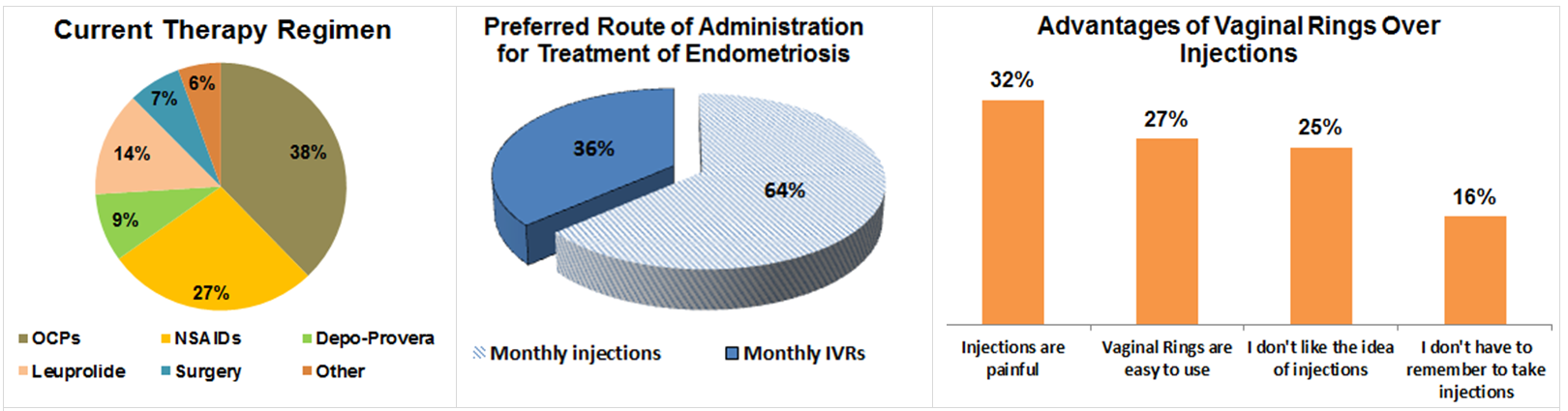
SURVEY RESULTS COMPARING WOMEN’S PREFERENCE FOR INTRAVAGINAL RINGS V/S INJECTIONS FOR ENDOMETRIOSIS
-In order to evaluate the potential market acceptance of a once-a-month intravaginal ring, in July 2015, we performed a research study with 262 endometriosis-affected women to understand their opinions on the acceptability of a GnRH agonist intravaginal ring.
-Ninety-five women (36%) with moderate to severe endometriosis-associated pain chose vaginal rings over injections.
-The current treatment for infertility often involves daily injections of Leuprolide under the skin of the abdomen for 14 consecutive days. We asked the 75 women suffering from infertility about their treatment preference between daily injections and an IVR. Thirty-five women (47%) women with infertility chose vaginal rings over injections.