ONE MONTH IN VITRO RELEASE OF ACICLOVIR INTO WATER
RESULTS OF A FIRST-IN-HUMAN CLINICAL TRIAL ASSESSING SAFETY AND PHARMACOKINETICS OF AN ACICLOVIR INTRAVAGINAL RING
We performed a first-in-human clinical trial to assess the tolerability and pharmacokinetics in six HIV-negative women with recurrent genital Herpes Simplex Virus (HSV). These women were asked to wear an aciclovir intravaginal ring for 7 days (n = 3) or 14 days (n = 3).
This first study in women of an aciclovir IVR demonstrated that genital tract levels of aciclovir that are similar to oral valaciclovir can be delivered without irritation, inflammation or systemic absorption.
The study also showed significant reductions in viral titer.
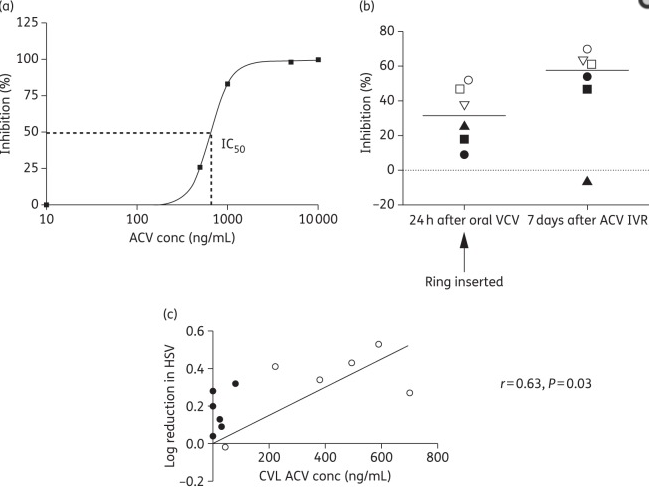
All six participants reported the ring to be very comfortable.The ring was well tolerated with no abnormal colposcopic findings or significant changes in inflammatory cytokine or chemokine concentrations in the cervicovaginal lavage.
