VITRASERT
The Vitrasert® implant was developed under Dr. Smith at his previous company, Control Delivery Systems, Inc. (now pSivida).
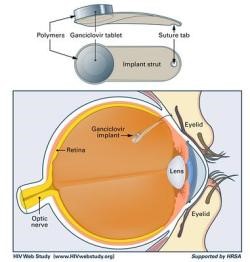
Prior to the approval of the ganciclovir implant Vitrasert® in 1996, the treatment of choice for cytomegalovirus (CMV) retinitis was intravenous ganciclovir, a treatment noted for its toxicity, inconvenience, expense, and relative ineffectiveness. After 1 year of IV ganciclovir therapy, 40% of patients develop viral resistance. The Vitrasert® intraocular device consists of a 4.5 mg pellet of ganciclovir coated by a laminated system of biocompatible polymers. It is surgically implanted into the vitreous through the pars plana and attached to the sclera by a suture. The superiority of the implant is due to local delivery (to avoid the problems of the blood eye barrier) and, more importantly sustained release delivery to avoid sub-therapeutic troughs.
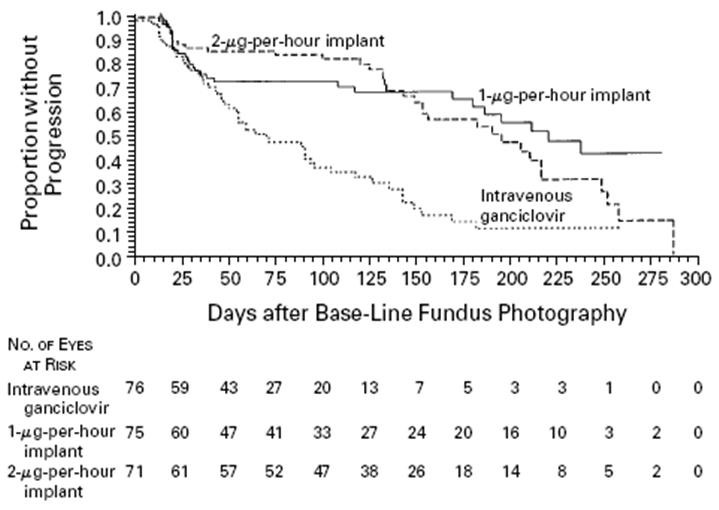
The Vitrasert® was compared to conventional IV dosing in 140 patients in a Phase III study. Experimental subjects were implanted with the device and control subjects received IV ganciclovir. The amount of ganciclovir used, compared to conventional dosing was reduced by a factor of 100,000, and the efficacy of treatment was significantly improved (p<0.0001 vs IV). Furthermore, after more than 15,000 devices have been implanted, the development of resistance during therapy has not been reported.
Following demonstration of efficacy of the ganciclovir implant in Phase 2 clinical studies a corporate alliance was forged with Chiron Corp, which funded the trials which led to the approval of the Vitrasert®. Due to commercial reasons, the Vitrasert is no longer marketed.
Retisert
The Retisert® implant was developed under Dr. Smith at his previous company, Control Delivery Systems, Inc. (now pSiveda).
Using fundamentally the same technology as Vitrasert®, we developed an intraocular sustained release steroid implant capable of maintaining anti-inflammatory intravitreal drug levels for periods of up to 3 years from a single implantation. This device was approved by the FDA in 2005 based on GMP production, release and stability studies; on GLP animal studies in multiple species demonstrating absence of toxicity, efficacy in models of inflammatory disease, and pharmacokinetics; and on two Phase III studies in patients with uveitis.
pSivida receives a royalty-based payment from Bausch & Lomb. The 2013 sales for Retisert® were $30M.
